Key points
- During chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. or a change of state, no atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. are created or destroyed. The total mass of chemicals before and after a reaction remains the same.
- This is called the Law of Conservation of Mass.
If you add 10 grams of sugar to 200 grams of warm water and stir to make a sugar solution, what mass of solution will you have?
10 grams of sugar plus 200 grams of water equals a total of 210 grams. Although you cannot see the dissolved sugar, all of the sugar particles and water particles you started with are still there.
What is the conservation of mass?
Video
Watch the video below to learn more about the conservation of mass.
Miss Roberts: So, today we're looking at the conservation of mass.
Katie: Oh, the conservation of mass. Where do we start with this one?
Miss Roberts: It sounds tricky, but it's easier if I just show you.
Katie: OK, great. Straight in with an experiment. This is my kind of science.
Miss Roberts: So I want you to put that bottle of water, please, on to the scales and tell me what it says in grams.
Katie: OK, it's says three hundred grams.
Miss Roberts: OK, so we are looking at the conservation of mass. Now, mass is the measure of how much stuff or matter is in an object and it's measured in either grams or kilograms.
Katie: Right, OK. So, this bottle of water has a mass of three hundred grams.
Miss Roberts: It does. And I want to show you what happens when a chemical reaction takes place or when there is a physical change in something that the mass stays the same. It is what's called 'conserved'.
Katie: OK. So, what's with the bottle of water, then? Are you just thirsty?
Miss Roberts: So, when you freeze water a physical change happens, because it turns from water to ice. But, the interesting thing is that although the water changes to ice the mass will stay the same. So, earlier I froze an identical bottle with three hundred grams of water. What do you see?
Katie: Well, what I see there is that it's increased. You know, the ice fills up more of the bottle. So, surely the mass has increased, proving science wrong?
Miss Roberts: Well, we'll see.
Katie: OK. Let's weigh it shall we?
Three hundred grams. It's exactly the same.
Miss Roberts: So, it may look like the mass has increased, because the ice takes up more room than the liquid water. But, the mass has in fact stayed the same.
Katie: What is this? Sorcery?
Miss Roberts: No, it's because the tiny particles that make up stuff or the matter in an object - known as atoms - they cannot be destroyed. So, when a physical change or a reaction takes place, atoms in a starting substance rearrange themselves and join back together to form a new substance. This is what we call a 'product'. But, the number of atoms will still be the same before and after reaction. So the total mass is the same.
Katie: That's amazing. So, when water freezes it makes the atoms rearrange themselves so the result is the ice. But, because atoms can't be destroyed, the ice still has the same amount of stuff in. It just looks completely different.
Miss Roberts: Exactly. So, in other words, the mass is conserved.
Katie: Wow. That's amazing. That's blown my mind, Miss Roberts. It really has. Here's a couple more examples to prove it too.
VOICEOVER: Zach has a bowl of ice cubes with a mass of five hundred grams. After some time the ice cubes melt, but the scales still show five hundred grams. This is because melting the ice has not created or destroyed any atoms and the mass has stayed the same. This is known as the law of conservation of mass. It applies to both chemical reactions and physical changes.
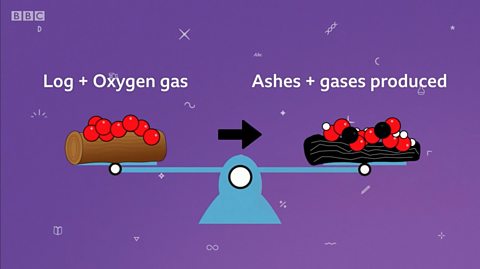
Zach puts a log on the fire. When it burns, Zac wonders if the ashes will be the same mass as the log was before. During burning, oxygen reacts with the log and releases carbon dioxide and water vapour - leaving solid ash. So, the mass of the ash is less than the loch. But, if we measured the mass of the gases produced and the ash, the total would be equal to the mass of the log and the oxygen used up during the combustion. This is because the mass of all the atoms will always stay the same.
Was the mass of the frozen water more than, less than, or the same as the mass of the liquid water?
The mass was the same in both. There was exactly the same number of atoms in the bottle. No atoms were created or destroyed, so the mass of the water/ice stayed the same.
What happens to mass?
Whenever a physical changeA reversible process which does not involve chemical bonds being broken or made. No new substances are made. or chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. happens, the mass of the chemicals before is the same as the mass of the chemicals after. This is called the Law of Conservation of Mass.
For example, if a piece of wood is burned, the mass of the log and oxygen used in the burning will be the same as the mass of the gases produced and the remaining ashes.
What is the difference between weight and mass?
Mass is the measurement of the amount of matter and is measured in kilograms or grams.
The weight of an object is the gravitational force between the object and the Earth. Weight is measured in newtons.
The weight of an object depends upon its mass and gravitational field strength. The mass of an object stays the same wherever it is in the universe, but its weight can change. For example, this happens if the object goes where the gravitational field strength is different from the gravitational field strength on Earth, such as into space or another planet.
Did you know?
The weight of an object on the moon is 1/6th of its weight on Earth.

Working scientifically
Maths skills for science
Maths skills are often used in science. We often need to make equations and do calculations.
In conservation of mass, the maths skills being used are addition and subtraction. The masses of the chemicals before the reaction can be added together. The total will be the same as the sum of the masses of the chemicals after the reaction. Subtraction will help you find the mass of a missing reactant.
How much carbon is needed to produce 88 g of carbon dioxide, if 64 g is oxygen?
88 g - 64 g = 24 g
Find out more about using maths when working scientifically.
The mass of a gas

It isn't easy to measure the mass of a gas, and it may seem as though gases don't weigh anything, but they do.
If 100 grams of water is put into a pan and boiled. Eventually, all the water will boil away as steam. If you collected all the steam and measured its mass, it would be exactly 100 grams.


If it looks like a physical change or a chemical reaction has lost mass, that is probably because gas has been produced and has escaped into the surrounding air.
What happens to the mass of a candle as it burns?
The mass of the candle will decrease. When the wax burns, it produces gases, carbon dioxide and water vapour. These gases then escape into the surrounding air.
Test your knowledge
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Chemical reactions
Find out more by working through a topic
- count8 of 12

- count9 of 12

- count10 of 12
- count11 of 12
