Key points
- Conductors are materials which allow electrical current to flow through them easily. Metals are generally good electrical conductors.
- Insulators are materials which are poor conductors and do not allow electrical current to flow through them easily.
- How well a material conducts depends on the number of free electronsAlso known as delocalised electrons. These electrons are not tightly bound to atoms, and are free to move through the material. A material with many free electrons is a good conductor. in the material.
Video - Conductors and insulators
Weтre surrounded by electrically powered devices these days. From our lights, to our kettles, and of course our phones!
Electricity flows through copper or aluminium wire to power or charge all of these things. Thatтs because metals are generally good electrical conductors
Thatтs one of the reasons that you should never touch exposed electrical wires!
Metals have lots of electrons, and some of them are free to move through the metal, so when theyтre connected in a circuit, the electrons can easily flow. This flow of electric charge is called a current.
Some metals are much better at conducting electricity than others.
Copper is a very good conductor.
And so is aluminium.
Whereas stainless steel conducts, but not quite as well.
Other materials can also conduct electricity, as long as they let the charge flow freely through them.
Like the graphite in a pencil.
Or this salty water.
Now, the outside of electric wires are usually covered in plastic because itтs a very poor conductor. It hardly conducts any electricity at all, so we can say itтs an electrical insulator.
Inside the plastic, all the electrons are tightly bound to the atoms, so the charges cannot move very far, and no current can flow.
And you can touch the wire and the outer casing of the electrical items without worrying about getting a shock.
Other insulators, which donтt allow electric current to flow through them, include wood and glass.
Although I can see why they chose plastic to wrap electrical wires. I wouldnтt fancy using a phone charger made of glass!
Can you answer these questions based on the video?
Are metals generally good conductors or poor conductors of electricity?
Why is the outside of electric wires generally covered in plastic?
Metals such as copper and aluminium are generally good conductors of electricity.
Plastic is a poor conductor of electricity as it is an electrical insulator. This means you donтt get an electric shock when you touch the plastic.
Conductors
Most metals allow electrical currentA flow of electrical charges (usually electrons) through a material. to flow through them easily because they have a low resistanceThis tells us how difficult it is for current to flow through a circuit. The more resistance there is, the harder it is for current to flow.. The process of electrical current flowing through a wire is called conductionThe process by which electrical current flows through a wire or another conductor., and materials which conduct are called conductors.
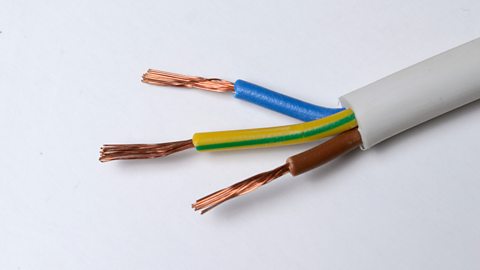
Structure of metals
To understand why metals conduct well, it is useful to look at the structure of a metalA substance which is usually shiny and a good conductor of electricity, eg copper, aluminium, gold, or silver. Most of the elements in the periodic table are metals..
Electrons usually orbit atoms. They cannot escape because the negatively charged electrons are attracted to the positively charged nucleus by strong electrostatic forces. The electrons are boundElectrons which are bound cannot move away from their atoms. Bound electrons cannot flow through a material. to the nucleus.
Because the outer electrons orbit further from the nucleus, the forces binding them are weaker. In a metal, the forces on these outer electrons are weak enough that the electrons can move relatively freely, from atom to atom. These are called free electrons or delocalised electrons.
Materials with many free electrons, such as metals, are good electrical conductors because these free electrons can flow through the metal when a potential difference (V)The energy transferred per unit charge. Potential difference is measure in volts (V). is applied.
Metals with more free electrons, like copper or silver, are the best conductors - they have the least resistance.
Metals with fewer free electrons, like lead or tin, have higher resistance so do not conduct as well.
Metals are made of neat rows of positive ions which are atoms which have lost their outer electrons. The arrangement is known as a crystal lattice, with a sea of free electrons that can move between them.
Insulators
Most non-metals, like plastic, glass and rubber do not allow electrical currentA flow of electrical charges (usually electrons) through a material. to flow through them easily. They have a high resistanceThis tells us how difficult it is for current to flow through a circuit. The more resistance there is, the harder it is for current to flow. and are called insulatorA material which does not allow electrical current to flow through it easily..
Non-metals are poor conductors because they have very few free electrons. This makes it difficult for electrical current to flow through them. Insulators such as plastic or rubber are used to cover electrical wires. This prevents electric shocks that could be caused if someone were to touch the bare wire.
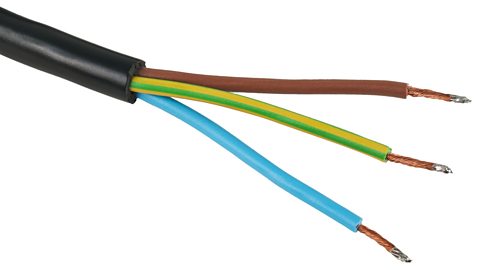
Mains electrical cables contain a metal wire, covered in plastic insulation. This ensures you cannot touch the bare wires which could cause a dangerous electric shock.


The case of the plug is usually made of plastic or rubber too, for the same reason.

Although most non-metals are poor conductors, graphite is an example of a non-metal which is a good electrical conductor. The centre of most pencils contains graphite. You could sharpen both ends of a pencil and use it to complete an electrical circuit, in place of a wire.
Conduction in liquids and gases
Liquids
Many liquids, such as distilled water (water, with no dissolved minerals or salts), oil or alcohol, are poor electrical conductors. These are called insulators.
Some liquids can conduct including:
rainwater
salt water
tap water
This is due to the dissolved salts and minerals they contain. Liquids which are electrical conductors are called electrolytes.

Gases
Gases are generally poor electrical conductors. This is why electrical currents cannot easily pass through the air.
However, electrical currents can pass through the air under certain conditions, for example when lightning strikes the ground, or an electrical spark jumps across a gap.
Find out more about electrostatic and shocks.

Test your knowledge
Quiz
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Electricity
Find out more by working through a topic
- count6 of 11

- count7 of 11

- count8 of 11
- count9 of 11
