The pH scale
The pH scale measures a solutionâs acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali).
- pH 0 â 2: strong acid, for example hydrochloric acid or sulfuric acid.
- pH 3 â 6: weak acid, for example ethanoic acid.
- pH 7: neutral
- pH 8 â 11: weak alkali, for example ammonia.
- pH 12 â 14: strong alkali, for example sodium hydroxide or potassium hydroxide.
Universal indicator solution or paper turns a range of colours, depending on the strength of the acidCorrosive substance which has a pH lower than 7. or alkaliA base which is soluble in water..
pH can be accurately measured using a pH meter or pH probe.
It gives a direct reading of the pH usually to one or two decimal places.
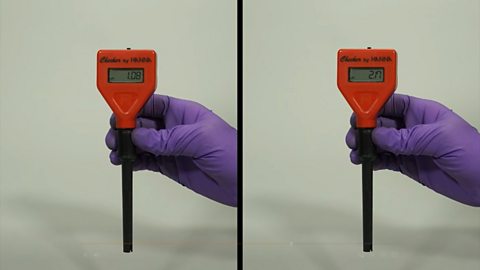
Two pH probes showing different readings.