Key points
- Combustion is another name for burning.
- In a combustion reaction, fuel is burned and reacts with oxygen to release energy.
Combustion activity
Play this game to see how changing the amount of fuel and oxygen affects how much energy is released.
What do petrol, natural gas, wood and coal have in common?
They are all fuels, which means that they can be burned to release energy.
Video
Watch the video below to find out what happens during a combustionAnother name for burning. When a fuel reacts with oxygen and releases useful energy. reaction.
While you are watching, think about the meanings of combustion, fuel and oxidation.
What is a combustion reaction?
Katie: Dr. Tim Gregory knows a thing or two about creating things that go BANG and BOOM.
So, given today's subject is all about chemical reactions, we couldn't be in better company. We're looking at combustion and oxidation in relation to chemical reactions. I mean, it sounds really exciting, sir. But, what do we actually need to to get started?
Dr. Tim: It is really exciting. This is one of my favourite subjects. So, combustion is a chemical reaction when a fuel is burned to release energy. A fuel is a substance which is burned to release energy in a useful way. And the final thing we need to know is oxidation. This is when a substance gains an oxygen atom in a reaction and this happens during combustion.
Katie: Um, excited. This really does sound like fun. Can you give us an example?
Dr. Tim: There are loads of examples of combustion, but I think it's best we explore this for now on a piece of paper. I'm going to show you the fire triangle. And this shows the three things that we need for a fire to start. The first thing we've got is a fuel and this is what we'd burn to release useful energy. The second thing we've got is oxygen and in the case of a fire we get the oxygen from the air. And the third thing that we need is to start a fire - we need a heat source.
These are the three things that we need to start a fire. And if just one of the sides is taken away from the fire triangle, the fire will not start. And, a fire that is already burning will quickly go out. And firefighting relies on these principles, so it's really important that we understand the science behind how fires work. The fire will go out when the fuel runs out, for example. But, it's often really unsafe to leave a fire for that long and so different types of fires need to be tackled in slightly different ways.
Katie: It's really interesting, sir. But, it's also so important to have this knowledge, isn't it? So that's why if you ever find yourself with a chip pan or oil fire, for example, you cover the pan with a damp cloth because that is removing the oxygen.
Dr. Tim: Absolutely. You remove the oxygen from the fire triangle and the chip pan fire will go out.
What are the three parts of the fire triangle?
A fuel, oxygen and heat are needed for a fire. If one of these is removed, a fire will go out.
What is a combustion reaction?
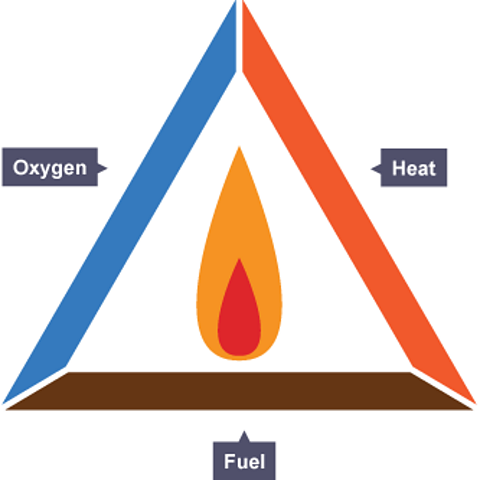
Combustion is another word for burning. In a combustion reaction, a fuelA substance which is burned to release energy. is heated and it reacts with oxygen.
The fire triangle summarises the three things needed for combustion - a fuel, heat and oxygen. If one of these things is removed from a fire, the fire goes out.
When fuels burn in combustion reactions, they release useful thermal energy (heat). Combustion reactions are used to heat our homes, power most cars, and to generate a lot of our electricity.

What is the difference between combustion and burning?
Nothing! They mean the same thing. Combustion is the scientific word for burning.
Oxidation reactions
An oxidationA type of chemical reaction when a substance reacts with oxygen. reaction is one where a substance reacts with oxygen and produces oxides. An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Combustion is an example of an oxidation reaction.

Many fuels contain carbon and hydrogen atoms. These fuels burn to produce oxides, carbon dioxide and water. Water, H₂O, is dihydrogen monoxide. Carbon dioxide, CO₂, is a greenhouse gas Gases such as water vapour, carbon dioxide, and methane in the Earth’s atmosphere that trap heat. that causes global warmingThe increase in Earth’s average temperature, caused mainly by the build-up of greenhouse gases in the atmosphere..


Water could also have the chemical name dihydrogen oxide or dihydrogen monoxide because two hydrogen atoms are bonded to one oxygen atom.
Petrol is a fuel which is used in cars. Petrol is made from molecules which contain only carbon and hydrogen atoms. What is produced when petrol burns in a car engine?
Carbon dioxide (COв‚‚) and water (Hв‚‚O) which are both oxides.
Working scientifically
Working safely with combustion in the lab
Combustion reactions can be investigated in a science lab.
If you are working with combustion it is important to work safely.
To work safely, you need to identify hazards eliminate them, or if this isn't possible, control the risk.
What safety precautions would you take to protect yourself when investigating combustion reactions?
- Wearing safety goggles
- Wearing protective gloves
- Wearing a protective lab coat.
- Tying back hair and tucking away loose clothing, such as ties.
These precautions will help reduce the risk of injury from the combustion investigation like getting burnt.
Find out more about how to work safely in science.
Combustion equations

Combustion reactions can be described using word and symbol equations.
The fuel used in gas barbeques is butane. Butane molecules contain only carbon atoms and hydrogen atoms and it is written as °дв‚„Hв‚Ѓвёц.
When the butane burns, the carbon atoms react with oxygen in the air and carbon dioxide, and the hydrogen atoms react with oxygen to make water.
The word equation for the combustion of butane is, butane + oxygen в†’ carbon dioxide + water.
The symbol equation for the combustion of butane is, \(2C_4H_{10} + 13O_2 \rightarrow 8CO_2 + 10H_2O\)
Like all combustion reactions, oxygen will always be one of the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow в†’. and be on the left of the equation. Most combustion reactions produce carbon dioxide and water, so these chemicals are written as the productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (в†’). on the right of the equation.

Charcoal is a fuel that contains carbon atoms but no hydrogen atoms. What is the word equation for the combustion reaction which occurs in a charcoal barbeque?
Carbon + oxygen в†’ carbon dioxide
Video
Watch this video to see how a combustion reaction can be represented with a word and symbol equation.
\(CH_4\) is the formula for methane, the largest component of the natural gas used by the oven.
This chef uses combustion daily when working with their oven
I have a gas igniter here which I use everyday so I don't burn my fingers, because when I use matches, that's what happens.
In the video, methane reacts with oxygen in a combustion reaction. What is the product?
The product of the reaction is carbon dioxide (COв‚‚) and water (Hв‚‚O).
Test your knowledge
Play the Atomic Labs game! gamePlay the Atomic Labs game!
Try out practical experiments in this KS3 science game.

More on Energy
Find out more by working through a topic
- count1 of 7
- count2 of 7

- count3 of 7

- count4 of 7