Exam practice
GCSE Chemistry: exam-style quiz by topic
Try this quiz based on GCSE Chemistry past papers. Choose the topic you would like to revise and answer the questions.
GCSE Chemistry: exam-style questions
OCR 21st Century Foundation and higher GCSE interactive tests based on past papers to get you ready for your chemistry exams. Topics include the periodic table, equations and more

GCSE Chemistry: quick-fire questions
Use our interactive quiz to understand how the OCR 21st Century foundation and higher chemistry GCSE exams work. Revise topics such as the periodic table and equations.

Quizzes
QUIZ: Electrolysis
This interactive quiz is for GCSE Chemistry (single science) students studying Electrolysis. Electrolysis involves using electricity to break down electrolytes to form elements.
QUIZ: Small molecules
This interactive quiz is suitable for GCSE Chemistry (single science) students studying small molecules. Test you knowledge of covalent bonds, electrons and atoms.

QUIZ: Nanoscience
This interactive quiz is for GCSE Chemistry (single science) students studying nanoscience. Answer questions about nanoparticles and the properties of Nanoparticulate materials.
QUIZ: Crude oil, hydrocarbons and alkanes
This interactive quiz is for GCSE Chemistry (single science) students studying crude oil, hydrocarbons and alkanes. Test your knowledge of fractional distillation and cracking.
QUIZ: Organic chemistry (1)
This interactive quiz is for GCSE Chemistry (single science) students studying organic chemistry. Test your knowledge of organic molecules, molecular formulae and their structures.
QUIZ: Organic chemistry (2)
This interactive quiz is for GCSE Chemistry (single science) students studying organic chemistry. Test your knowledge of carboxylic acids, polymerisation and amino acids.
QUIZ: Water
This interactive quiz is for GCSE Chemistry (single science) students studying water. Test your knowledge of potable water, desalination and waste water treatment.

QUIZ: Ways of reducing the use of resources
This interactive quiz is for GCSE Chemistry (single science) students studying the ways of reducing the use of resources. Test your knowledge of the life-cycle assessment.
Podcasts
Atomic structure and the periodic table
Learn about atomic structure and the periodic table for your GCSE chemistry exam, with Dr Sunayana Bhargava and Tulela Pea.

Bonding, structure and properties
Learn about bonding, structure and properties of matter for your GCSE chemistry exam, with Dr Sunayana Bhargava and Tulela Pea.

Chemical changes
Dr Sunayana Bhargava and Tulela Pea take you through what you need to know about chemical changes for your GCSE chemistry exam.

Science exam techniques
Learn all about science exam techniques for your GCSE science exams with Dr Alex Lathbridge.

Air and water
Formulae and equations - OCR 21st Century
Chemists use symbols and formulae to represent elements, ions and compounds. Word equations and balanced chemical equations model the changes that happen in chemical reactions.
Earth's atmosphere
The Earth, its oceans and atmosphere are made of elements and compounds in different states. The particle model may be used to explain the different properties of the solid, liquid and gas states.

Energy changes in chemical reactions
Exothermic reactions give out energy and the temperature increases. Endothermic reactions take in energy and the temperature decreases.
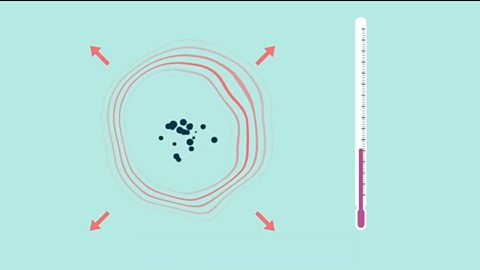
Chemical cells
A chemical cell produces a potential difference until one of the reactants is used up. A hydrogen-oxygen fuel cell uses hydrogen and oxygen, and water is the only product.

The evidence for climate change
Human activity is causing an increase in greenhouse gases. The Earth’s climate is changing and most scientists agree that the two are connected.

Improving the supply of potable water
As the Earth’s population increases, the demand for potable water increases. Climate change could make finding water sources harder. The treatment needed to make the water safe depends upon the source.

Sample exam questions - air and water - OCR 21st Century
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Chemical patterns
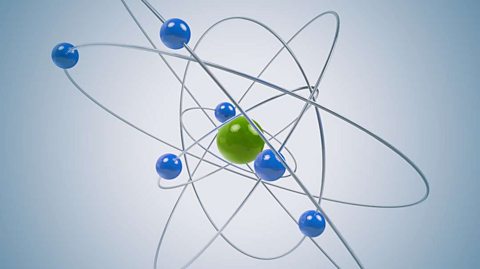
The evolution of the atom
The atomic model consists of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of particles in an atom can be calculated from its atomic number and mass number.
Periodic table of elements
Mendeleev made an early periodic table. In the modern periodic table, elements are arranged in order of atomic number in periods and groups. Electronic arrangements model how electrons are arranged.

Metals, non-metals and compounds
In this OCR GCSE Chemistry study guide, we'll go through the group 0 elements, the noble gases, are all unreactive non-metal gases. We'll explain how they show trends in their physical properties and how their uses depend on their inertness, low density and non-flammability.

Using equations to represent chemical reactions
All substances are described by their formulae, which are used to write balanced chemical equations. Writing the formula for an ionic compound requires knowledge of the charges on its ions.
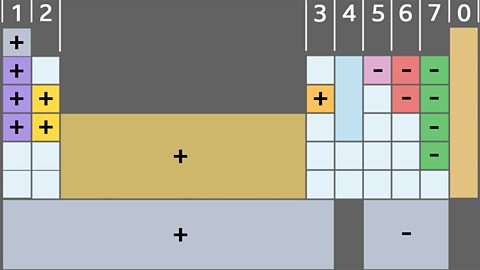
Properties of transition metals
Transition metals and their compounds are used in a wide range of goods and as catalysts in industry. The properties of individual transition metals determine which should be used for what purpose.

Sample exam questions - chemical patterns
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Chemicals of the natural environment
Atomic structure of metals
Metals have characteristic properties which make them very useful. These can be explained using appropriate models of metallic structure and bonding.

Extracting metals with different reactivities
Metals can be arranged in order of reactivity by observing their reactions with water, acid and displacement reactions with other metal compounds. These reactions determine how it is extracted from its ore.

Electrolytes and electrolysis
Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. Metal ions and non-metal ions are attracted to opposite electrodes.

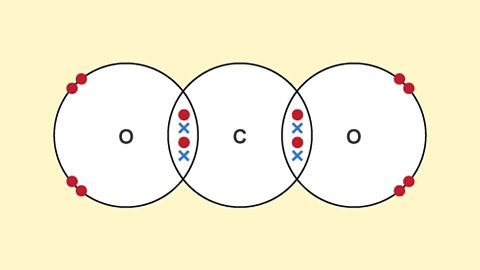
Covalent bonds
A covalent bond is a shared pair of electrons. Covalent bonding results in the formation of molecules. Simple molecular substances have low melting and boiling points, and do not conduct electricity.
Crude oil
Crude oil is a finite resource. It can be separated into its useful fractions by fractional distillation. Some fractions undergo cracking to help meet demand for the smaller molecules.
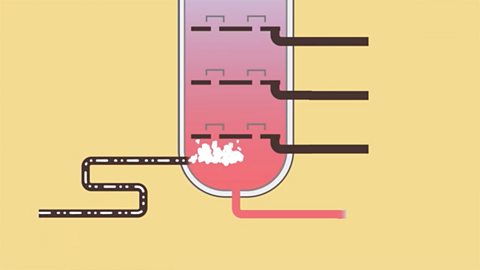
Organic chemistry - OCR 21st Century
The alkanes form a homologous series of saturated hydrocarbons. Other homologous series are based on the same carbon chain but with the addition of a different functional group.

Sample exam questions - chemicals of the natural environment
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple-choice, structured, mathematical and practical questions.

Material choices
Using data to choose materials
There is a wide range of materials, including glass and clay ceramics, polymers, metals and composite materials. They have different physical properties, making them suitable for different uses.

Alloys and polymers
Alloys are mixtures of metals that have useful properties. Addition polymers are made from molecules containing C=C bonds. DNA, starch and proteins are biological polymers.

Bonding, structure and properties of materials - OCR 21st Century
Different materials can be made from atoms of the same element. The properties of the material depend upon the bonding between the atoms and structure of the substance.
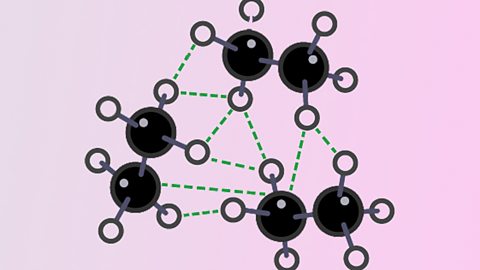
Uses of nanoparticles
Nanoparticles are 1 nm to 100 nm in size. They have very large surface area to volume ratios. The properties of nanoparticulate substances are different from those of the same substance in bulk.
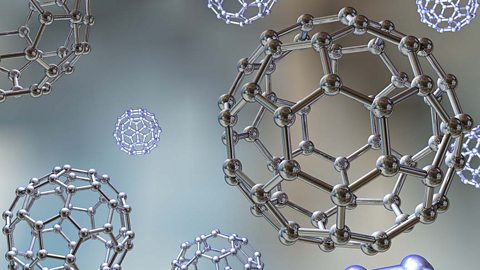
Corrosion - OCR 21st Century
Many metals oxidise when exposed to air. If exposure continues a metal can become weaker over time. This sort of corrosion may be prevented by a range of methods.

Product disposal and recycling
A life-cycle assessment provides a 'cradle-to-grave' analysis of the impact of a manufactured product on the environment. The method of disposal of a product can affect impact on the environment.

Sample exam questions - Material choices - OCR 21st Century
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Chemical analysis
Separating chemical mixtures
There are different ways to separate mixtures, for example by filtration, crystallisation, distillation or chromatography. The method chosen depends upon the type of mixture.

Finding the composition of unknown samples
Quantitative analysis is used by chemists to make measurements and calculations. Alkalis neutralise acids to make salts and water. Their concentration can be analysed using titrations.

Calculating amounts of substances
No atoms are created or destroyed during a chemical reaction. This allows calculations to be made on the quantities of substances that react and of the products made.
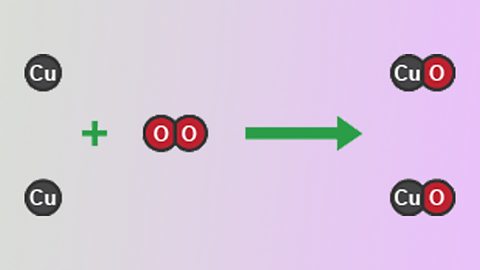
Calculating yields - OCR 21st Century
The percentage yield shows how much product is obtained compared to the maximum possible mass. Molar gas volume can be used to make calculations about reactions between gases.

Measuring the amounts of chemicals in solutions
Quantitative analysis is used by chemists to make measurements and calculations. Alkalis neutralise acids to make salts and water. Their concentration can be analysed using titrations.

Sample exam questions - chemical analysis
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Making useful chemicals
Making useful products from acids
Many products require ingredients made by the reaction of acids. These include cleaning products and food additives. Acids are used on an industrial scale to produce chemicals such as fertilisers.

Controlling the rate of reactions
Rate of reaction is a measure of how fast a reaction takes place. In industry, chemists control rates of reaction to ensure the production is safe but still fast enough to keep up with demand.

Factors affecting the yield of chemical reactions
In a reversible reaction, the products can react to produce the original reactants. The conditions chosen for an industrial reaction are related to producing an acceptable yield in an acceptable time.

Making chemicals on an industrial scale
In industrial reactions, an acceptable yield in an acceptable time is needed. Catalysts increase the rate of the desired reaction, which helps to reduce the energy demand and cost of a process.

Sample exam questions - making useful chemicals
Understanding how to approach exam questions helps to boost exam performance. Question types will include multiple choice, structured, mathematical and practical questions.

Ideas about science
Making scientific observations
In general, scientists work out explanations based on repeatedly collecting and analysing data. The way in which scientists develop explanations is known as the scientific method.

Drawing conclusions from data
Scientific data is collected, presented and then analysed. It is important to evaluate the quality of data before drawing a conclusion.

Scientific explanations
Data shows a correlation if the change in a factor is similar to the change in an outcome. Scientists must find a scientific explanation to conclude that a particular factor causes an outcome.

The impact of science and technology on society
It is important that scientists communicate their work with the public, other scientists and politicians. This means decisions can be made that consider the risks, benefits, costs and ethical issues.

Practical skills
Scientific observation
Scientific investigations have several stages - planning, collecting data, analysing data and evaluation. It is important to understand how to carry out each stage of the investigation.

Links
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link
- External linkExternal link
- External linkExternal link
- SubscriptionSubscription
- External linkExternal link